Role of APS reductase in biogeochemical sulfur isotope fractionation
Min Sub Sim, Hideaki Ogata, Wolfgang Lubitz, Jess F. Adkins, Alex L. Sessions, Victoria J. Orphan & Shawn E. McGlynn
Nature Communications volume 10, Article number: 44 (2019)
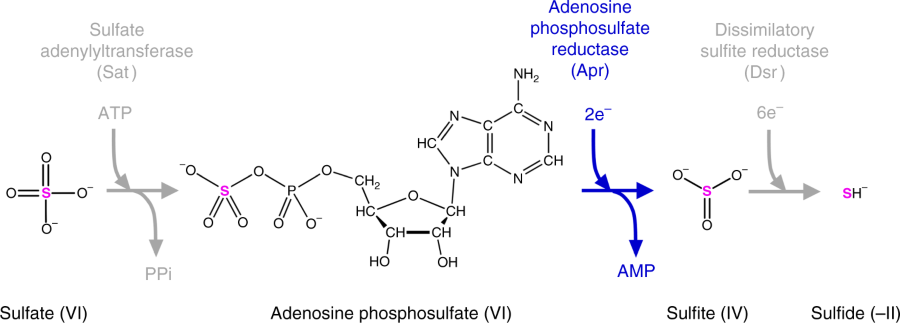
The pathway of dissimilatory sulfate reduction with the molecular structures of sulfur-containing metabolites and the enzymes catalyzing their transformation. Adenosine phosphosulfate reductase, the enzyme of interest in this study, breaks the first S–O bond in the sulfate group, which involves the transfer of two electrons
Abstract
Sulfur isotope fractionation resulting from microbial sulfate reduction (MSR) provides some of the earliest evidence of life, and secular variations in fractionation values reflect changes in biogeochemical cycles. Here we determine the sulfur isotope effect of the enzyme adenosine phosphosulfate reductase (Apr), which is present in all known organisms conducting MSR and catalyzes the first reductive step in the pathway and reinterpret the sedimentary sulfur isotope record over geological time. Small fractionations may be attributed to low sulfate concentrations and/or high respiration rates, whereas fractionations greater than that of Apr require a low chemical potential at that metabolic step. Since Archean sediments lack fractionation exceeding the Apr value of 20‰, they are indicative of sulfate reducers having had access to ample electron donors to drive their metabolisms. Large fractionations in post-Archean sediments are congruent with a decline of favorable electron donors as aerobic and other high potential metabolic competitors evolved.
Acknowledgments
This work was supported by the Research Resettlement Fund for the new faculty of Seoul National University to M.S.S., the NASA Research Opportunities in Space and Earth Sciences grant award number NNX14AO48G to S.E.M. and V.J.O., JSPS KAKENHI Grant Number 10751084 to S.E.M., and the Gordon and Betty Moore Foundation Grant GBMF 3306 to V.J.O. and A.L.S. This research was a part of the project titled ‘Understanding the deepsea biosphere on seafloor hydrothermal vents in the Indian Ridge (20170411)’, funded by the Ministry of Oceans and Fisheries, Korea. We are grateful for insightful and helpful conversations with Boswell A. Wing, David T. Johnston, David A. Fike, and Itay Halevy. We are especially grateful to Tatsuhiko Yagi and Yoshiki Higuchi for helping with initiating collaboration.
Author information
Affiliations
School of Earth and Environmental Sciences, Seoul National University, Seoul, 08826, South Korea
Min Sub Sim
Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA, 91125, USA
Min Sub Sim, Jess F. Adkins, Alex L. Sessions, Victoria J. Orphan & Shawn E. McGlynn
Max Planck Institute for Chemical Energy Conversion, Stiftstrasse 34-36, D-45470, Mülheim an der Ruhr, Germany
Hideaki Ogata & Wolfgang Lubitz
Institute of Low Temperature Science, Hokkaido University, Sapporo, 060-0819, Japan
Hideaki Ogata
Earth-Life Science Institute, Tokyo Institute of Technology, Ookayama, Tokyo, 152-8550, Japan
Shawn E. McGlynn
Contributions
M.S.S. and S.E.M. devised the study. H.O. and W.L. purified APS reductase, and M.S.S. executed enzymatic assay and sulfur isotope measurements. M.S.S. and S.E.M. wrote the first draft of the manuscript, and J.F.A., A.L.S., and V.J.O. contributed to interpretation and writing.
Competing interests
The authors declare no competing interests.
Corresponding authors
Correspondence to Min Sub Sim or Shawn E. McGlynn.
FREE PDF GRATIS: Nature Communications Sup. Info.


