This is an unedited manuscript that has been accepted for publication. Nature Research are providing this early version of the manuscript as a service to our customers. The manuscript will undergo copyediting, typesetting and a proof review before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers apply.
Insights into the assembly and activation of the microtubule nucleator γ-TuRC
Peng Liu, Erik Zupa, Annett Neuner, Anna Böhler, Justus Loerke, Dirk Flemming, Thomas Ruppert, Till Rudack, Christoph Peter, Christian Spahn, Oliver J. Gruss, Stefan Pfeffer & Elmar Schiebel
Nature (2019)
Abstract
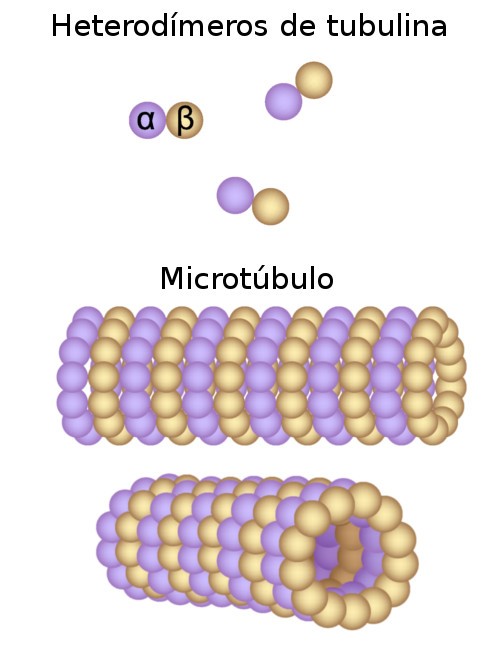
Microtubules (MTs) are dynamic polymers of αβ-tubulin and play critical roles in cell signaling, cell migration, intracellular transport processes and chromosome segregation1. They assemble de novo from α/β-tubulin dimers in an essential process termed MT nucleation. Complexes containing the protein γ-tubulin serve as structural templates for the MT nucleation reaction2. In vertebrates, MTs are nucleated by the 2.2 MDa γ-tubulin ring complex (γ-TuRC) composed of γ-tubulin, five related γ-tubulin complex proteins (GCP2-6) and additional factors3. GCP6 is unique among the GCP proteins, because it carries an extended insertion domain of unknown function. High-resolution structural information on the γ-TuRC is not available, strongly limiting our understanding of MT formation in cells and tissue2. Here, we present the cryo-EM structure of γ-TuRC from Xenopus laevis at 4.8 Å global resolution, revealing a 14-spoked arrangement of GCPs and γ-tubulins in a partially flexible open left-handed spiral with a uniform sequence of GCP variants (Fig. 1a). Via specific interactions with other GCP proteins, the GCP6-specific insertion domain scaffolds the assembly of the γ-TuRC. Unexpectedly, we identified Actin as a bona fide structural component of γ-TuRC with functional relevance in MT nucleation. The γ-TuRC spiral geometry is suboptimal for MT nucleation and a controlled conformational rearrangement of the γ-TuRC is required for its activation. Collectively, our cryo-EM reconstruction provides unprecedented insights into the molecular organization, the assembly and the activation mechanism of vertebrate γ-TuRC and will serve as an important framework for the mechanistic understanding of fundamental biological processes associated with MT nucleation, e.g. meiotic and mitotic spindle formation and centriole biogensis 4.
+++++
Subscription or payment needed/Requer assinatura ou pagamento: Nature
+++++
Professores, pesquisadores e alunos de universidades públicas e privadas com acesso ao site Portal de Periódicos CAPES/MEC podem ler este artigo da Nature gratuitamente e mais 33.000 publicações científicas.


